Africa Pharmacovigilance Dashboard
Mastercard Foundation and Africa CDC launched the Saving Lives and Livelihoods programme in June 2021. Through this ground-breaking initiative, a team of implementing partners, including Akros Research, AMREF, African Society for Laboratory Medicine (ASLM), Infectious Disease Institute (IDI), International Federation of the Red Cross and Red Crescent Socities (IFRC), UNICEF, and WFP work directly with African government counterparts to stregthen delivery, integration, and safety of Covid19 vaccinations. These efforts are laying the groundwork for enhanced pandemic preparedness within the government workforce and at Africa CDC and well as future vaccine manufacturing in Africa.
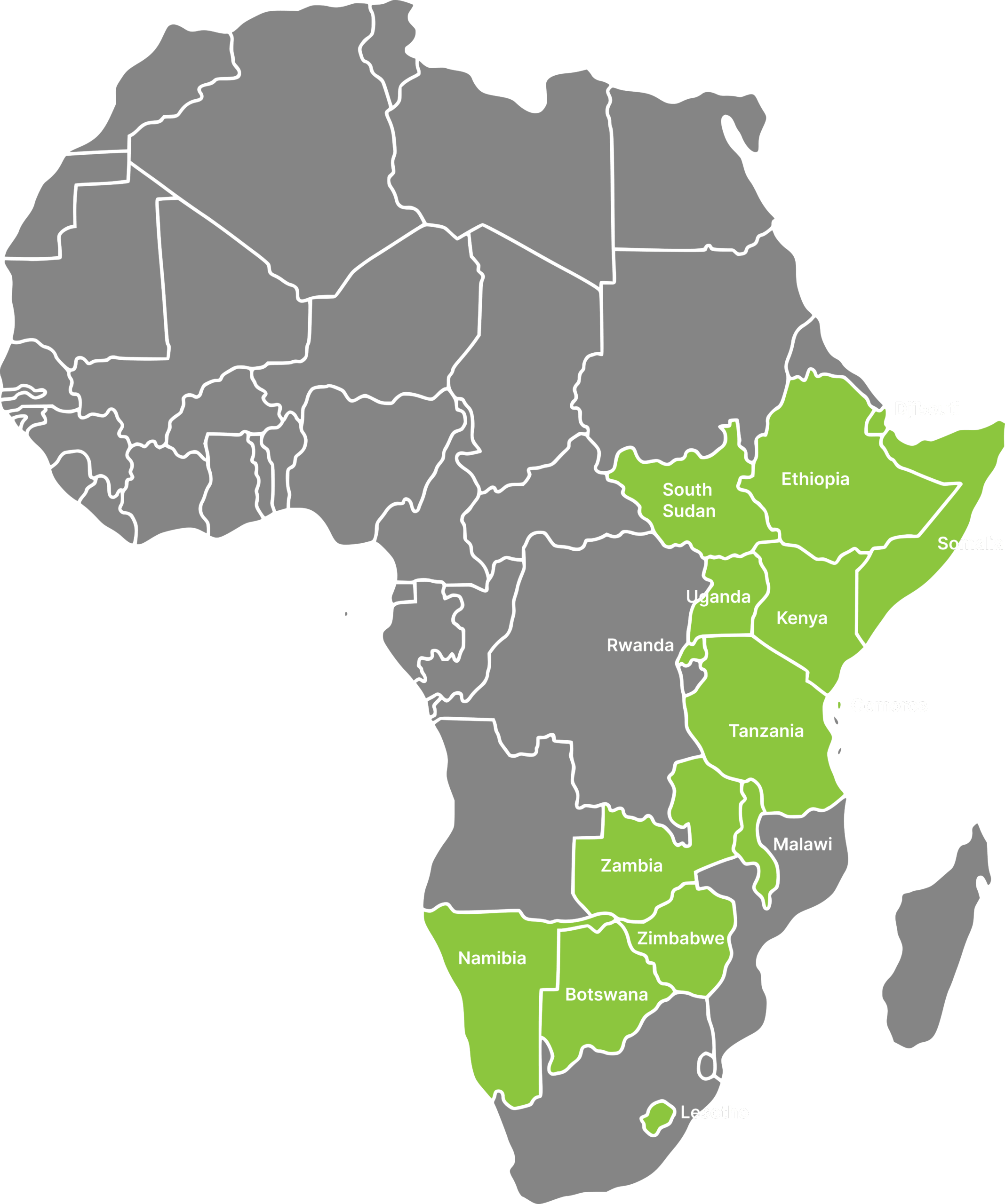
Now in its second phase of programme implementation, Akros Research is directly supporting safety surveillance strengthening efforts at regional- and country-levels across Eastern and Southern Africa. Fifteen african member states have benefitted from health workforce training, expert technical assistance to design or enhance standard operating procedures, and direct support stakeholders workint to increase adverse events following immunization (AEFI) reporting, investigation, and causality assessment, as mapped below.
Akros Research – Saving Lives and Livelihoods (SLL) Program

The Saving Lives and Livelihoods (SLL) initiative is a partnership between the Africa Centres for Disease Control and Prevention (Africa CDC) and the Mastercard Foundation, launched in June 2021. Implemented in two phases, Phase I (June 2022 – July 2023) and Phase II (October 2024 – December 2025), the initiative aims to strengthen Africa’s health systems by enhancing COVID-19 vaccination efforts, building community-based health workforces, integrating pandemic response into routine health systems, and improving the Africa CDC’s capacity for long-term resilience.
Akros Research serves as one of the implementing partners, leading pharmacovigilance (PV) and vaccine safety surveillance strengthening across 15 African Union Member States, nine in Eastern Africa (Comoros, Djibouti, Ethiopia, Kenya, Rwanda, Somalia, South Sudan, Tanzania, and Uganda) and six in Southern Africa (Botswana, Lesotho, Malawi, Namibia, Zambia, and Zimbabwe). This work is carried out in close collaboration with national Ministries of Health, National Regulatory Authorities (NRAs), and Expanded Program on Immunization (EPI) teams.
Weak pharmacovigilance systems in Africa limit the continent’s ability to detect, assess, and respond to adverse events following immunization (AEFI) or of special interest (AESI). The SLL initiative addresses key challenges such as limited funding for PV systems, fragmented data platforms, and low awareness on adverse event reporting.
Through the SLL initiative, Akros is advancing four key objectives:
- Develop a Real-Time AEFI/AESI Reporting Framework:
Establish a region-wide digital platform enabling member states to report safety data to Africa CDC, providing visibility into adverse event trends and improving decision-making.
- Strengthen National PV Center Capacity:
Apply a tiered support model based on country maturity levels, ranging from foundational reporting system setup to advanced analytics and backlog clearance, to build reliable, data-driven PV systems.

- Enhance Networks and Collaboration:
Strengthen regional PV networks and promote knowledge exchange through collaboration with WHO (AfroVigil), ISoP, The Global Health Network (TGHN), Medicine Control Authority of Zimbabwe (MCAZ) and national centers. This includes hosting webinars, peer learning sessions, and developing visualization tools such as Tableau PV dashboards.
- Develop the Pharmacovigilance Framework for Action:

Create a continental framework to guide PV strengthening across Africa, aligning with the African Medicines Agency to improve regulatory oversight, safety monitoring, and the use of real-world data and AI for evidence generation.
Between March and September 2025, Akros has supported all 15 countries in implementing tailored PV microplans and tracking progress through key indicators such as developing a regional reporting platform, member states reporting their AEFI data to the Africa CDC, people trained, serious AEFIs investigated, causality assessment meetings held, creation of the PV tableau dashboard, PV webinars hosted and development of the PV Framework for Action.
As SLL Phase II concludes in December 2025, Akros will finalize all member state activities, hand over the Africa CDC AEFI/AESI reporting platform, publish the PV dashboard, update regional PV network websites, and deliver the Pharmacovigilance Framework for Action, cementing a sustainable foundation for vaccine safety surveillance and public health resilience across Africa.
