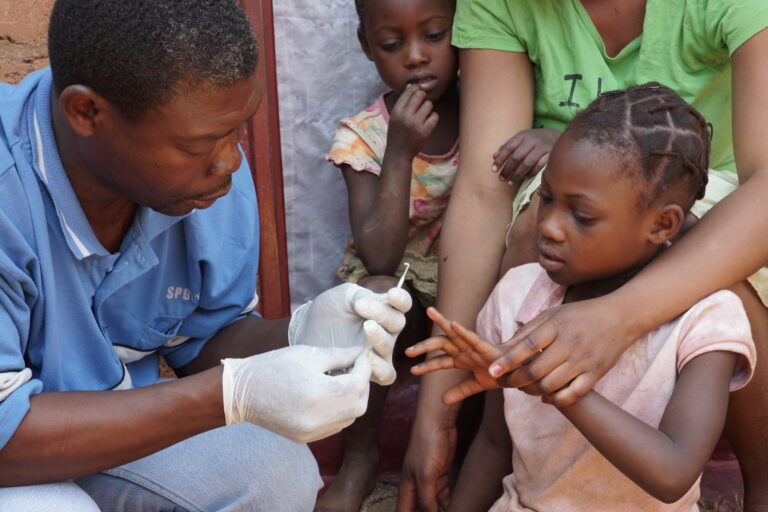
Kigali, Rwanda – September 2025

Akros Research staff, Africa CDC representative and workshop participants at Onomo Hotel Kigali in September 2025 during the Objective 1 workshop for East Africa member states.
The Saving Lives and Livelihoods (SLL) program is a flagship initiative of the Africa Centres for Disease Control and Prevention (Africa CDC), supported by the Mastercard Foundation. Launched in June 2021, SLL aims to ensure vaccine equity for more than 65 million people across Africa while strengthening routine immunization and pandemic preparedness. It also contributes to the Foundation’s broader Youth in Work strategy, which focuses on creating meaningful employment opportunities for young people in Africa’s health sector.
The SLL program was implemented in a two phase approach. Phase I (June 2021 – July 2023) focused on rapid vaccine deployment and workforce mobilization, while Phase II (October 2024 – December 2025) builds on those gains by integrating COVID-19 vaccination into routine health systems, strengthening community health workforces, and advancing sustainable pandemic preparedness efforts.
As part of Phase II, Africa CDC has partnered with several organizations, including Akros Research, which is leading efforts to strengthen pharmacovigilance and safety surveillance across 15 member states, nine in East Africa and six in Southern Africa. Since Phase I, Akros has been developing a real-time reporting platform for Adverse Events Following Immunization (AEFI) and Adverse Events of Special Interest (AESI). The platform, designed in collaboration with Africa CDC, will enable countries to detect, document, and respond to vaccine safety signals efficiently, supporting a continent-wide approach to vaccine safety.
Akros has made significant progress in enhancing Pharmacovigilance systems in East and Southern Africa, and also completed the development of the Africa CDC AEFI/AESI reporting platform. To accelerate the rollout of this reporting tool, Akros Research recently hosted a three-day regional training workshop in Kigali, Rwanda, bringing together pharmacovigilance experts and immunization program representatives from East African Member States. The workshop provided hands-on training on the new Africa CDC AEFI/AESI reporting platform and guided participants through technical troubleshooting on DHIS2 and OneDrive integrations, tested data uploads, and data cleaning and validation exercises.
According to the Africa CDC representative, Dr Lenny Kamau Gitundu, Senior Country Representative for Rwanda,
“The Africa CDC AEFI Reporting Platform workshop marks an important milestone in strengthening vaccine safety surveillance across the continent. By bringing together national experts to test and refine this regional platform, we are laying the foundation for a robust, real-time pharmacovigilance system. This collective effort ensures that data on vaccine safety is transformed into actionable insights that protect communities and reinforce public trust in immunization”
Participants also shared sample datasets from Vigiflow to test and refine the system further. These collaborative sessions strengthened regional ownership and prepared Member States for upcoming implementation activities.
Reflecting on the workshop, Dr M’Haza Hassane Head of the Pharmacovigilance Department, National Agency for Medicines and Medical Evacuations in Comoros, noted at the end of the training workshop on the Africa CDC Adverse Event Notification Platform (MAPI), held in Kigali:
“This training has been a valuable opportunity to strengthen our capacities in pharmacovigilance and vaccine safety surveillance. The AEFI platform is very user-friendly and easy to use. Once operational, it will significantly contribute to data management at the national level, and even across Africa CDC, by promoting closer collaboration among Member States.
I am confident that the skills and tools acquired during this workshop will help strengthen pharmacovigilance in the Comoros and, more broadly, reinforce the resilience of our health systems in the region”
Akros plans to conduct a similar workshop for Southern African Member States in Botswana in November 2025, after which the tool will be migrated to Africa CDC’s infrastructure. This marks a key transition toward full ownership and management of the platform by Africa CDC. In the interim, Akros will continue to refine the system using real datasets and feedback from participating countries.
Through these collective efforts, Africa CDC, the Mastercard Foundation and Akros are laying the groundwork for a stronger, more coordinated vaccine safety surveillance network in Africa, one that safeguards public health and ensures the continent remains prepared for future health challenges.